Just two days after the FDA announced plans to recommend that the DEA schedule 7-hydroxymitragynine (7-OH) as a controlled substance, on July 31, council members in Toledo, Ohio announced a possible ban on all of kratom, as well as legislation that would urge the state to ban as well.
The ban in Toledo would only include a ban on the sale of kratom. Possession would remain legal. Ohio law already prohibits kratom in capsule, liquid, or food-infused forms, allowing only natural dried leaf or powder. Enforcement of this law is apparently lax enough that the Toledo-Lucas County Health Department has announced it is “actively embargoing kratom products found to be in violation of state regulations.”
Toledo city council debated these actions on Wednesday August 20. Representatives from the local rehabilitation industry attended in support of the ban, and a representative of the Global Kratom Coalition (GKC) testified against the ban, but in favor of regulations. Walker Gallman told WTOL11, “Regulation is definitely the answer here. What I would recommend is a 21 age requirement, labeling requirements, manufacturing standards, restrictions on marketing the children.”
Toledo has a population of over 260,000 with 516,000 residents in the greater metropolitan area. A low estimate for kratom consumers in this area (about 1%) would be about 5100 residents. At least dozens of retail stores that sell kratom within city limits would be affected by a ban.
Citing public safety, the legislation was introduced by Councilman Sam Melden and co-sponsored by Vanice Williams, John Hobbs III, and Theresa Morris. There has been no vocal opposition from other council members. The same sponsors have also introduced companion legislation that would urge the Ohio Board of Pharmacy (BOP) to classify kratom as a Schedule I controlled substance statewide.
In 2018, the Ohio BOP proposed to classify kratom as a Schedule I controlled substance, citing kratom’s potential for abuse, lack of accepted medical use, and alleged public health risks. This sparked intense public backlash, led primarily by the American Kratom Association (AKA). Thousands of Ohio residents mobilized in opposition, submitting written comments and testifying at public hearings. Advocates challenged the Board’s claims about kratom’s dangers, especially the assertion that six Ohio deaths were directly attributable to kratom—pointing out that these cases involved poly-drug use or underlying medical conditions. The pushback was swift and well-organized. Faced with overwhelming resistance and mounting scrutiny of its evidence base, the Board ultimately delayed its scheduling decision in August 2019, marking a significant victory for consumer advocates and setting the stage for future regulatory approaches.
In 2021 the Ohio legislature passed HB236. The bill established a legal framework for the manufacture and sale of kratom products, while explicitly prohibiting the BOP from scheduling kratom as a controlled substance. Instead of criminalizing possession, HB 236 placed oversight under the Department of Agriculture, requiring processors to obtain licenses and adhere to strict labeling and testing standards. The legislation also capped the allowable concentration of 7-hydroxymitragynine at 2%, aiming to limit the potency of extracts while preserving access to natural leaf products. Eventually, the Department of Agriculture interpreted the law to only allow plain leaf kratom, and prohibiting capsules, liquids, edibles, vapes, extracts, and any isolated or concentrated 7-hydroxymitragynine products.
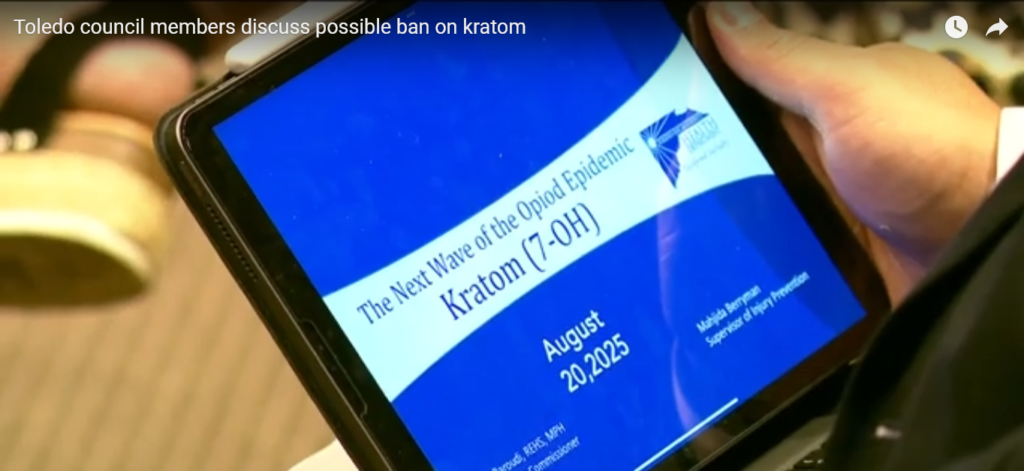
It’s also unclear if Toledo city council members or the Toledo-Lucas County Health Department understand the difference between 7-OH and kratom, or is purposely trying to conflate the two. A screen cap (below) from the WTOL11 report shows the title screen of a presentation by Toledo-Lucas County Health Department Commissioner Karim Baroudi entitled “The Next Wave of the Opioid Epidemic: Kratom (7-OH)”.